A12 Hayer-Hartl/Hartl: Mechanisms of chaperone-mediated protein folding and assembly
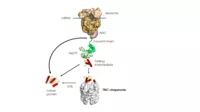
A substantial fraction of newly-synthesised proteins require assistance from molecular chaperones to reach their folded states efficiently and at a biologically relevant time scale. In the second funding period, we used a combination of spectroscopic methods and cryo-electron microscopy to analyse the mechanism of the eukaryotic chaperonin TRiC/CCT in promoting protein folding and characterised the protein Hgh1 as a new chaperone that cooperates with TRiC. We now plan to investigate the function of the major chaperone Hsp70 in protein folding. We will use biophysical techniques such as spFRET and H/DX-MS to investigate whether Hsp70, like the chaperonins, can accelerate folding and, if so, by which mechanism.