B03 Sattler: Conformational control of multi-domain RNA binding proteins
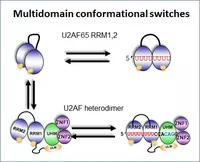
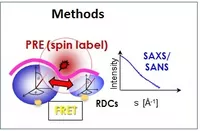
Structural mechanisms and dynamics of multidomain protein complexes during early spliceosome assembly will be investigated. Building on our recent results, we will study conformational switches during recognition of the 3’ splice site RNA (involving the essential splicing factors U2AF2/1, hnRNP A1 and SF1), the interaction of these factors with U1 snRNP at the 5’ splice site via bridging factors in complex E, and the protein complex involved in recognition of neuronal microexons. Integrative structural biology in solution, state-of-the-art NMR spectroscopy, SAXS/SANS and FRET experiments will be combined with crystallography and cryo-EM. Effects of RNA sequence variations, conformational equilibria, intrinsically disordered regions and disease-linked mutations in the splicing factors will be characterised. The results will unravel how conformational regulation and dynamics of multidomain protein complexes affects RNA recognition and the regulation of pre-mRNA splicing during spliceosome assembly.